Varicose Vein Treatment
Medical Adhesive
(VenaSeal™ Closure System)
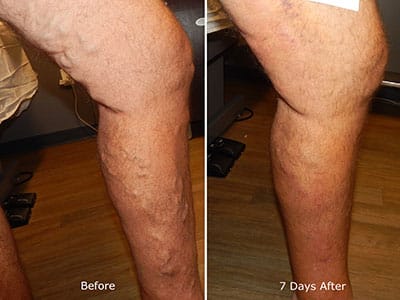
The VenaSeal closure procedure is a revolutionary new varicose vein treatment and is the only FDA approved procedure to use medical adhesive (medical grade super glue - cyanoacrylate) that seals the veins. Once the diseased vein has been glued shut with cyanoacrylate, it will undergo a process of hardening (referred to as sclerosis), blood is immediately re-routed through other healthy veins in the leg and the affected varicose vein will be gradually absorbed by the body. This unique approach is very safe and minimally invasive. Most patients convey that, "it is a painless vein treatment." At our vein center, we have experienced 100% success using VenaSeal with zero complaints of pain.
The VenaSeal system is truly “state of the art” in treatment to close the vein. This procedure eliminates the need for multiple injections of a local anesthetic (tumescent anesthesia) and is as effective as thermal based ablation but does not involve the use of heat. For these reasons, medical adhesive is the most “pain free” venous treatment available to date. A small amount of anesthetic at the entry point is all that is required. Beyond that, patients will not feel any pain or discomfort.
Yelp Review Jilanne Rose
"Jilanne changed my life. My legs look and feel better than I could have ever expected. I recommend her highly for any procedure you may need. She is also very caring, communicative and goes beyond to make you feel comfortable during procedures. Thank you Jilanne!"
Michel G., Scottsdale, AZ
Advanced Vein Institute Patient
Healthgrades Review Jilanne Rose
"My experience with Dr. Rose was exceptional. I had 3 procedures and I thought I'd have pain and major discomfort. I did not have ANY pain or discomfort. The team was professional, knowledgeable and courteous. I didn't have to wait one time upon arrival. I couldn't have been more pleased with the entire experience. Both my legs feel so great! Thank you Dr. Rose and your team for all your care and concern. Well done!"
Jane K. in San Tan Valley
Advanced Vein Institute Patient
Medicare Approved Payment For VenaSeal Vein Treatment
This is very exciting news! Effective January 1, 2018, Medicare covers this procedure. Jilanne is a medical adhesive vein closure expert and is working with Medtronic in order to help gain coverage for patients with private insurances during the months following Medicare's coverage launch.
Varicose Veins Clinical Trials
The use of cyanoacrylate adhesive for treatment has been meticulously trialed in the United States and Europe over several years. Results published show a low risk of complications and excellent closure results. The VeClose 36-month follow-up data was recently published in Phlebology. This publication helps support the clinical effectiveness and utility, QoL, and safety outcomes of cyanoacrylate adhesive for the treatment of chronic venous insufficiency. A copy of the publication is available at: https://journals.sagepub.com/doi/full/10.1177/0268355518810259.
View the VenaSeal 36-month clinical research results.
Unlike other varicose vein treatments, there is rapid (if not immediate) return to normal activities and minimal to no bruising. Compression stockings are not required following this procedure and walking immediately afterward is encouraged. Normal daily activity can be resumed right away. Patients have expressed that their night leg cramps and leg pain resolves very quickly, often with the ability to sleep without awaking, the same day of treatment. For individuals who have not had a solid night sleep in years because of leg surgery fear, this is certainly a welcome in-office alternative to fix painful leg veins.
Technical VenaSeal Overview
The VenaSeal™ closure system is the only non-tumescent, non-thermal, non-sclerosant treatment for symptomatic venous reflux in the United States. The VenaSeal™ closure system is a medical device designed to be used as a system and is indicated for the permanent closure of lower extremity superficial truncal veins, such as the great saphenous vein (GSV), through endovascular embolization with coaptation. This system is intended for use in adults with clinically symptomatic venous reflux as diagnosed by duplex ultrasound (DUS). The VenaSeal™ adhesive, an n-butyl-2-cyanoacrylate (n-BCA) based formulation, is a clear, free-flowing liquid that is provided sterile following exposure to dry heat. This adhesive polymerizes in the vessel via an anionic mechanism (i.e., VenaSeal adhesive begins to polymerize into a solid material upon contact with body fluids or tissue). This acute coaptation halts blood flow through the insufficient vein until the implanted adhesive becomes fibrotically encapsulated to establish a durable, chronic occlusion of the treated vein.[1] The VenaSeal™ delivery system components facilitate the placement and delivery of VenaSeal adhesive within the target vessel. The VenaSeal delivery system components include a catheter, introducer, dilator, dispenser gun, dispenser tips, 3 cc syringes, and 0.035 in J-wire guidewire.
Both time to closure and recanalization rate were lower with this system than with radiofrequency ablation. Specific benefits of this closure system include high level of efficacy (95.3% closure rate at 2 years[2]), rapid return to normal activity and work (0.2 days for VenaSeal9 vs. 3.6 days for endovenous laser[3] and 4.3 days for foam sclerotherapy9), elimination of the risk/concerns of lidocaine toxicity, allergy with tumescent anesthesia and of thermal nerve injury, absence of venous thrombosis, no requirement for post-procedure compression stockings, and greatly improved patient comfort due to elimination of multiple needle sticks, and associated bruising.
The VenaSeal™ system is intended to be used by a licensed physician experienced in the use of high resolution ultrasound imaging. The procedure, catheter insertion, glue injection, compression and occlusions, is typically performed in an office or other outpatient setting. Medtronic offers a comprehensive physician training package to ensure VenaSeal™ is delivered effectively and consistent outcomes are achieved, including two supervised training sessions, as directed by the FDA.
Clinical Efficacy
The primary clinical study (VeClose) was a controlled, randomized, prospective, multicenter, pivotal study in which patients with venous reflux in the great saphenous vein (GSV) were treated with either the VenaSeal™ system or radiofrequency ablation (RFA) therapy. The study’s primary objective was to demonstrate safety and effectiveness of the VenaSeal system for the treatment of lower extremity truncal reflux compared to RFA therapy using a legally marketed device with similar indications for use.8 Patients were treated between March 11, 2013 and September 11, 2013, with 242 patients (including 20 roll-in patients) enrolled at 10 investigational sites. Following treatment, subjects were followed at 3 days, and 1, 3, 6, 12, 24, and 36 months. No adjunctive treatments were permitted until after the 3-month follow-up visit.
VeClose complete closure rates of targeted GSV at specified timeframes, including results from recently published 24-month and 36-month data show the effective closure rate of 95.3% and 94.4% for cyanoacrylate compared to 94% and 91.9% for RFA, respectively.
The prospective study and other publications measured QoL improvement by three assessments: Venous clinical Severity Score (VCSS), a clinical venous disease assessment, the Aberdeen Varicose Vein Questionnaire (AVVQ) and EQ-5D – both of which provide patient-reported quality of life scores. In all three assessments, patients treated with the VenaSeal™ closure system reflected statistically significant improvement from baseline:
- VCSS demonstrated statistically significant improvement out to 6 months and sustained through 12, 24, and 36-month time points. No significant difference between VenaSeal™ closure system and RFA at 36 months.
- AVVQ subjects experienced statistically significant improvement from baseline and improvement (decreasing total AVVQ score) over time through 36 months. No significant difference between VenaSeal™ closure system and RFA at 36 months.
- EQ-5D subjects experienced statistically significant improvement from baseline and improvement over time through 36 months. No significant difference between VenaSeal™ closure system and RFA at 36 months.
Clinical studies demonstrate the safety and effectiveness of the VenaSeal procedure, which is administered without the use of tumescent anesthesia, thus avoiding multiple needle sticks that result in patient discomfort. Other advantages of this procedure include:[4],[5],[6]
- Reduced recovery time and quick return of patients to their normal activities following the procedure
- Little to no pain, both during and after the procedure
- Little or no bruising, following the procedure
- Only a very small amount of VenaSeal™ adhesive is used to close the vein.
Clinical Utility
VenaSeal™ was released in the U.S. in 2015 following FDA approval. Since then early adopters, both patients and physicians, have added to the growing base of overwhelming clinical benefits of this therapy even in a self-pay model. Approximately 39,152 VenaSeal™ closure systems have been sold in the U.S. since 2015, of which 33,281 (85%) were sold following Category 1 CPT codes and positive Medicare coverage in 2018.
There are 5 prospective studies including 16 published articles of clinical trials on VenaSeal™ supporting the effectiveness and durability of cyanoacrylate closure for the treatment of venous reflux disease.
[1] VenaSeal™ closure system [Instructions for Use]. Medtronic Inc., February 2015. Accessed September 28, 2018.
[2] Gibson K, Ferris B. Cyanoacrylate closure of incompetent great, small and accessory saphenous veins without the use of post-procedure compression: Initial outcomes of a post-market evaluation of the VenaSeal System (the WAVES Study). Vascular [serial online]. April 2017;25(2):149-156.
[3] Rasmussen L, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg [serial online]. August 2011;98(8):1079-1087.
[4] Experience the VenaSeal™ closure system. Medtronic. http://medtronicendovenous.com/patients/7-2-venasealclosure-procedure/. Accessed September 28, 2018.
[5] Experience the VenaSeal™ closure system. Medtronic. http://medtronicendovenous.com/patients/7-2-venasealclosure-procedure/3-2-5-venaseal-closure-procedure-faqs/. Accessed September 28, 2018.
[6] Morrison N, Gibson K, McEnroe S, et al. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). J Vasc Surg. 2015;61(4):985-994.


